Monoferric
What is Monoferric (Derisomaltose)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The prognosis of patients undergoing spinal deformity surgery is often compromised by perioperative anemia due to iron deficiency. The aim of this randomized, controlled trial was to evaluate whether postoperative ferric derisomaltose intravenous injection may improve anemia and prognosis in patients undergoing spinal deformity surgery comparing with oral iron. Participants will be randomly assign...
Summary: POAM is a multicenter, randomized, controlled, internal pilot trial, using a conventional, parallel group, two-armed design at 3 cardiac surgery centres in Canada. The study is designed to assess the feasibility of a future, definitive RCT investigating whether, in patients with chronic iron-deficiency anemia undergoing cardiac surgery, IV iron therapy in the postoperative period (initiated shortl...
Summary: Double blind, placebo controlled, multicenter randomized trial in pregnant women in the U.S. (N=300) to test the central hypothesis that IV iron in pregnant women with IDA (Hb\<11 g/dL and ferritin\<30 ng/mL) at 13 - 30 weeks will be effective, safe and cost-effective in reducing severe maternal morbidity-as measured by maternal anemia at delivery-and will also improve offspring neurodevelopment.
Related Latest Advances
Brand Information
- who have intolerance to oral iron or have had unsatisfactory response to oral iron
- who have non-hemodialysis dependent chronic kidney disease (NDD-CKD)
- Injection: 1,000 mg iron/10 mL (100 mg/mL) single-dose vial
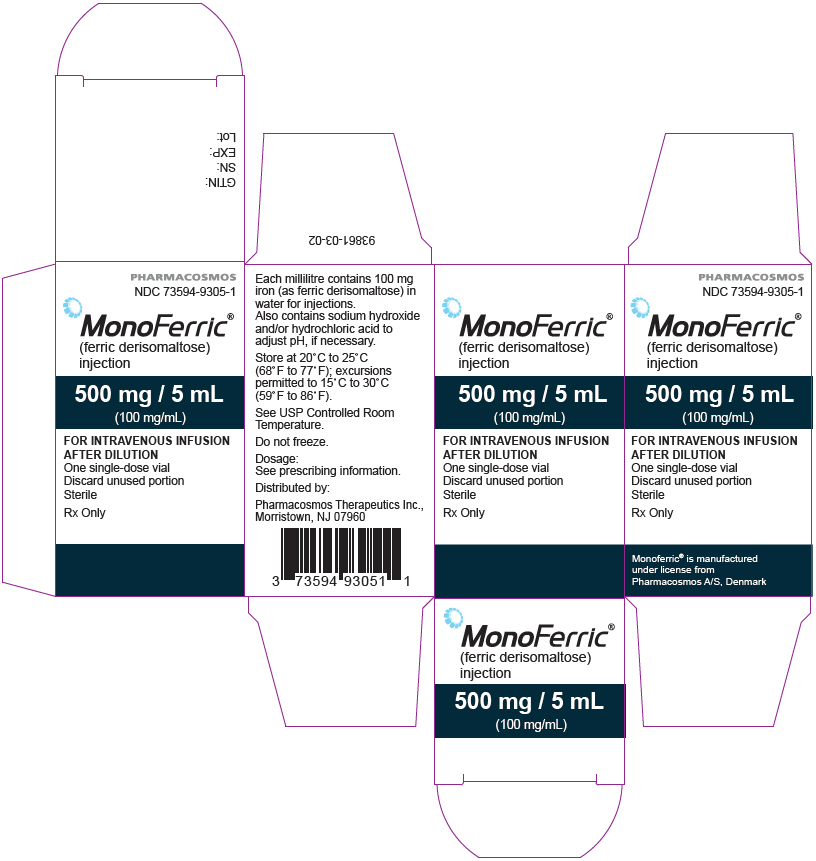
- Injection: 500 mg iron/5 mL (100 mg/mL) single-dose vial
- Injection: 100 mg iron/mL single-dose vial
- Hypersensitivity Reactions
- Iron Overload