Generic Name
LamoTRIgine
Brand Names
Lamictal, Lamictal ODT, Subvenite
FDA approval date: January 17, 1995
Classification: Anti-epileptic Agent
Form: Tablet, Kit
What is Lamictal (LamoTRIgine)?
Lamotrigine is indicated for: Epilepsy — adjunctive therapy in patients aged 2 years and older: Partial-onset seizures. primary generalized tonic-clonic seizures. generalized seizures of Lennox-Gastaut syndrome.
Approved To Treat
Top Global Experts
There are no experts for this drug
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
There is no clinical trials being done for this treatment
Related Latest Advances
There is no latest advances for this treatment
Brand Information
LAMICTAL (lamotrigine)
WARNING: SERIOUS SKIN RASHES
LAMICTAL XR can cause serious rashes requiring hospitalization and discontinuation of treatment. The incidence of these rashes, which have included Stevens-Johnson syndrome, is approximately 0.8% (8 per 1,000) in pediatric patients (aged 2 to 16 years) receiving immediate-release lamotrigine as adjunctive therapy for epilepsy and 0.3% (3 per 1,000) in adults on adjunctive therapy for epilepsy. In a prospectively followed cohort of 1,983 pediatric patients (aged 2 to 16 years) with epilepsy taking adjunctive immediate-release lamotrigine, there was 1 rash-related death. LAMICTAL XR is not approved for patients younger than 13 years. In worldwide postmarketing experience, rare cases of toxic epidermal necrolysis and/or rash-related death have been reported in adult and pediatric patients, but their numbers are too few to permit a precise estimate of the rate.
The risk of serious rash caused by treatment with LAMICTAL XR is not expected to differ from that with immediate-release lamotrigine. However, the relatively limited treatment experience with LAMICTAL XR makes it difficult to characterize the frequency and risk of serious rashes caused by treatment with LAMICTAL XR.
Other than age, there are as yet no factors identified that are known to predict the risk of occurrence or the severity of rash caused by LAMICTAL XR. There are suggestions, yet to be proven, that the risk of rash may also be increased by (1) coadministration of LAMICTAL XR with valproate (includes valproic acid and divalproex sodium), (2) exceeding the recommended initial dose of LAMICTAL XR, or (3) exceeding the recommended dose escalation for LAMICTAL XR. However, cases have occurred in the absence of these factors.
Nearly all cases of life-threatening rashes caused by immediate-release lamotrigine have occurred within 2 to 8 weeks of treatment initiation. However, isolated cases have occurred after prolonged treatment (e.g., 6 months). Accordingly, duration of therapy cannot be relied upon as means to predict the potential risk heralded by the first appearance of a rash.
Although benign rashes are also caused by LAMICTAL XR, it is not possible to predict reliably which rashes will prove to be serious or life threatening. Accordingly, LAMICTAL XR should ordinarily be discontinued at the first sign of rash, unless the rash is clearly not drug related. Discontinuation of treatment may not prevent a rash from becoming life threatening or permanently disabling or disfiguring
1DOSAGE AND ADMINISTRATION
LAMICTAL XR extended-release tablets are taken once daily, with or without food. Tablets must be swallowed whole and must not be chewed, crushed, or divided.
1.1General Dosing Considerations
Rash
There are suggestions, yet to be proven, that the risk of severe, potentially life-threatening rash may be increased by (1) coadministration of LAMICTAL XR with valproate, (2) exceeding the recommended initial dose of LAMICTAL XR, or (3) exceeding the recommended dose escalation for LAMICTAL XR. However, cases have occurred in the absence of these factors
The risk of nonserious rash may be increased when the recommended initial dose and/or the rate of dose escalation for LAMICTAL XR is exceeded and in patients with a history of allergy or rash to other AEDs.
LAMICTAL XR Patient Titration Kits provide LAMICTAL XR at doses consistent with the recommended titration schedule for the first 5 weeks of treatment, based upon concomitant medications, for patients with partial-onset seizures and are intended to help reduce the potential for rash. The use of LAMICTAL XR Patient Titration Kits is recommended for appropriate patients who are starting or restarting LAMICTAL XR
It is recommended that LAMICTAL XR not be restarted in patients who discontinued due to rash associated with prior treatment with lamotrigine unless the potential benefits clearly outweigh the risks. If the decision is made to restart a patient who has discontinued LAMICTAL XR, the need to restart with the initial dosing recommendations should be assessed. The greater the interval of time since the previous dose, the greater consideration should be given to restarting with the initial dosing recommendations. If a patient has discontinued lamotrigine for a period of more than 5 half-lives, it is recommended that initial dosing recommendations and guidelines be followed. The half-life of lamotrigine is affected by other concomitant medications
LAMICTAL XR Added to Drugs Known to Induce or Inhibit Glucuronidation
Because lamotrigine is metabolized predominantly by glucuronic acid conjugation, drugs that are known to induce or inhibit glucuronidation may affect the apparent clearance of lamotrigine. Drugs that induce glucuronidation include carbamazepine, phenytoin, phenobarbital, primidone, rifampin, estrogen-containing products, including oral contraceptives, and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir. Valproate inhibits glucuronidation. For dosing considerations for LAMICTAL XR in patients on estrogen-containing products including contraceptives and atazanavir/ritonavir, see below and Table 1 and Table 5.
Target Plasma Levels
A therapeutic plasma concentration range has not been established for lamotrigine. Dosing of LAMICTAL XR should be based on therapeutic response
Women Taking Estrogen-Containing Oral Contraceptives
Starting LAMICTAL XR in Women Taking Estrogen-Containing Oral Contraceptives: Although estrogen-containing oral contraceptives have been shown to increase the clearance of lamotrigine [see Clinical Pharmacology (, no adjustments to the recommended dose-escalation guidelines for LAMICTAL XR should be necessary solely based on the use of estrogen-containing oral contraceptives. Therefore, dose escalation should follow the recommended guidelines for initiating adjunctive therapy with LAMICTAL XR based on the concomitant AED or other concomitant medications (see Table 1). See below for adjustments to maintenance doses of LAMICTAL XR in women taking estrogen-containing oral contraceptives.
Adjustments to the Maintenance Dose of LAMICTAL XR in Women Taking Estrogen-Containing Oral Contraceptives:
(1) Taking Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, the maintenance dose of LAMICTAL XR will in most cases need to be increased by as much as 2-fold over the recommended target maintenance dose to maintain a consistent lamotrigine plasma level [see Drug Interactions (.
(2) Starting Estrogen-Containing Oral Contraceptives: In women taking a stable dose of LAMICTAL XR and not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, the maintenance dose will in most cases need to be increased by as much as 2-fold to maintain a consistent lamotrigine plasma level [see Drug Interactions (. The dose increases should begin at the same time that the oral contraceptive is introduced and continue, based on clinical response, no more rapidly than 50 to 100 mg/day every week. Dose increases should not exceed the recommended rate (see Table 1) unless lamotrigine plasma levels or clinical response support larger increases. Gradual transient increases in lamotrigine plasma levels may occur during the week of inactive hormonal preparation (pill-free week), and these increases will be greater if dose increases are made in the days before or during the week of inactive hormonal preparation. Increased lamotrigine plasma levels could result in additional adverse reactions, such as dizziness, ataxia, and diplopia. If adverse reactions attributable to LAMICTAL XR consistently occur during the pill-free week, dose adjustments to the overall maintenance dose may be necessary. Dose adjustments limited to the pill-free week are not recommended. For women taking LAMICTAL XR in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, no adjustment to the dose of LAMICTAL XR should be necessary [see Drug Interactions (.
(3) Stopping Estrogen-Containing Oral Contraceptives: In women not taking carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, the maintenance dose of LAMICTAL XR will in most cases need to be decreased by as much as 50% in order to maintain a consistent lamotrigine plasma level [see Drug Interactions (. The decrease in dose of LAMICTAL XR should not exceed 25% of the total daily dose per week over a 2-week period, unless clinical response or lamotrigine plasma levels indicate otherwise [see Clinical Pharmacology (. In women taking LAMICTAL XR in addition to carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation, no adjustment to the dose of LAMICTAL XR should be necessary [see Drug Interactions (.
Women and Other Hormonal Contraceptive Preparations or Hormone Replacement Therapy
The effect of other hormonal contraceptive preparations or hormone replacement therapy (HRT) on the pharmacokinetics of lamotrigine has not been systematically evaluated. Other estrogen-containing therapies, such as HRT, may interfere with lamotrigine. Therefore, close clinical monitoring on effectiveness of LAMICTAL XR with dose adjustment may be necessary
Patients Taking Atazanavir/Ritonavir
While atazanavir/ritonavir does reduce the lamotrigine plasma concentration, no adjustments to the recommended dose-escalation guidelines for LAMICTAL XR should be necessary solely based on the use of atazanavir/ritonavir. Dose escalation should follow the recommended guidelines for initiating adjunctive therapy with LAMICTAL XR based on concomitant AED or other concomitant medications (see Tables
Patients with Hepatic Impairment
Experience in patients with hepatic impairment is limited. Based on a clinical pharmacology study in 24 subjects with mild, moderate, and severe liver impairment, the following general recommendations can be made
Patients with Renal Impairment
Initial doses of LAMICTAL XR should be based on patients’ concomitant medications (see
Discontinuation Strategy
For patients receiving LAMICTAL XR in combination with other AEDs, a re-evaluation of all AEDs in the regimen should be considered if a change in seizure control or an appearance or worsening of adverse reactions is observed.
If a decision is made to discontinue therapy with LAMICTAL XR, a step-wise reduction of dose over at least 2 weeks (approximately 50% per week) is recommended unless safety concerns require a more rapid withdrawal
Discontinuing carbamazepine, phenytoin, phenobarbital, primidone, or other drugs such as rifampin and the protease inhibitors lopinavir/ritonavir and atazanavir/ritonavir that induce lamotrigine glucuronidation should prolong the half-life of lamotrigine; discontinuing valproate should shorten the half-life of lamotrigine.
1.2Adjunctive Therapy for Primary Generalized Tonic-Clonic and Partial-Onset Seizures
This section provides specific dosing recommendations for patients aged 13 years and older. Specific dosing recommendations are provided depending upon concomitant AEDs or other concomitant medications.
1.3Conversion from Adjunctive Therapy to Monotherapy
The goal of the transition regimen is to attempt to maintain seizure control while mitigating the risk of serious rash associated with the rapid titration of LAMICTAL XR.
To avoid an increased risk of rash, the recommended maintenance dosage range of LAMICTAL XR as monotherapy is 250 to 300 mg given once daily.
The recommended initial dose and subsequent dose escalations for LAMICTAL XR should not be exceeded
Conversion from Adjunctive Therapy with Carbamazepine, Phenytoin, Phenobarbital, or Primidone to Monotherapy with LAMICTAL XR
After achieving a dose of 500 mg/day of LAMICTAL XR using the guidelines in
The regimen for the withdrawal of the concomitant AED is based on experience gained in the controlled monotherapy clinical trial using immediate-release lamotrigine.
Conversion from Adjunctive Therapy with Valproate to Monotherapy with LAMICTAL XR
The conversion regimen involves the 4 steps outlined in
Conversion from Adjunctive Therapy with Antiepileptic Drugs other than Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate to Monotherapy with LAMICTAL XR
After achieving a dosage of 250 to 300 mg/day of LAMICTAL XR using the guidelines in
1.4Conversion from Immediate-Release Lamotrigine Tablets to LAMICTAL XR
Patients may be converted directly from immediate-release lamotrigine to LAMICTAL XR extended-release tablets. The initial dose of LAMICTAL XR should match the total daily dose of immediate-release lamotrigine. However, some subjects on concomitant enzyme-inducing agents may have lower plasma levels of lamotrigine on conversion and should be monitored
Following conversion to LAMICTAL XR, all patients (but especially those on drugs that induce lamotrigine glucuronidation) should be closely monitored for seizure control
2CONTRAINDICATIONS
LAMICTAL XR is contraindicated in patients who have demonstrated hypersensitivity (e.g., rash, angioedema, acute urticaria, extensive pruritus, mucosal ulceration) to the drug or its ingredients
3ADVERSE REACTIONS
The following serious adverse reactions are described in more detail in the
- Serious Skin Rashes
- Hemophagocytic Lymphohistiocytosis
- Multiorgan Hypersensitivity Reactions and Organ Failure
- Cardiac Rhythm and Conduction Abnormalities
- Blood Dyscrasias
- Suicidal Behavior and Ideation
- Aseptic Meningitis
- Withdrawal Seizures
- Status Epilepticus
3.1Clinical Trial Experience with LAMICTAL XR for Treatment of Primary Generalized Tonic-Clonic and Partial-Onset Seizures
Most Common Adverse Reactions in Clinical Trials
Adjunctive Therapy in Patients with Epilepsy: Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In these 2 trials, adverse reactions led to withdrawal of 4 (2%) patients in the group receiving placebo and 10 (5%) patients in the group receiving LAMICTAL XR. Dizziness was the most common reason for withdrawal in the group receiving LAMICTAL XR (5 patients [3%]). The next most common adverse reactions leading to withdrawal in 2 patients each (1%) were rash, headache, nausea, and nystagmus.
Table 4 displays the incidence of adverse reactions in these two 19-week, double-blind, placebo-controlled trials of patients with PGTC and partial onset seizures.
Adverse reactions were also analyzed to assess the incidence of the onset of an event in the titration period, and in the maintenance period, and if adverse reactions occurring in the titration phase persisted in the maintenance phase.
The incidence for many adverse reactions caused by treatment with LAMICTAL XR was increased relative to placebo (i.e., treatment difference between LAMICTAL XR and placebo ≥2%) in either the titration or maintenance phases of the trial. During the titration phase, an increased incidence (shown in descending order of percent treatment difference) was observed for diarrhea, nausea, vomiting, somnolence, vertigo, myalgia, hot flush, and anxiety. During the maintenance phase, an increased incidence was observed for dizziness, tremor, and diplopia. Some adverse reactions developing in the titration phase were notable for persisting (>7 days) into the maintenance phase. These persistent adverse reactions included somnolence and dizziness.
There were inadequate data to evaluate the effect of dose and/or concentration on the incidence of adverse reactions because, although patients were randomized to different target doses based upon concomitant AEDs, the plasma exposure was expected to be generally similar among all patients receiving different doses. However, in a randomized, parallel trial comparing placebo with 300 and 500 mg/day of immediate-release lamotrigine, the incidence of the most common adverse reactions (
Monotherapy in Patients with Epilepsy: Adverse reactions observed in this trial were generally similar to those observed and attributed to drug in adjunctive and monotherapy immediate-release lamotrigine and adjunctive LAMICTAL XR placebo-controlled trials. Only 2 adverse events, nasopharyngitis and upper respiratory tract infection, were observed at a rate of >3% and not reported at a similar rate in previous trials. Because this trial did not include a placebo control group, causality could not be established [see Clinical Studies (.
3.2Other Adverse Reactions Observed during the Clinical Development of Immediate-Release Lamotrigine
All reported reactions are included except those already listed in the previous tables or elsewhere in the labeling, those too general to be informative, and those not reasonably associated with the use of the drug.
Adjunctive Therapy in Adults with Epilepsy
In addition to the adverse reactions reported above from the development of LAMICTAL XR, the following adverse reactions with an uncertain relationship to lamotrigine were reported during the clinical development of immediate-release lamotrigine for treatment of epilepsy in adults. These reactions occurred in ≥2% of patients receiving immediate-release lamotrigine and more frequently than in the placebo group.
Body as a Whole: Headache, flu syndrome, fever, neck pain.
Musculoskeletal: Arthralgia.
Nervous: Insomnia, convulsion, irritability, speech disorder, concentration disturbance.
Respiratory: Pharyngitis, cough increased.
Skin and Appendages: Rash, pruritus.
Urogenital (female patients only): Vaginitis, amenorrhea, dysmenorrhea.
Monotherapy in Adults with Epilepsy
In addition to the adverse reactions reported above from the development of LAMICTAL XR, the following adverse reactions with an uncertain relationship to lamotrigine were reported during the clinical development of immediate-release lamotrigine for treatment of epilepsy in adults. These reactions occurred in >2% of patients receiving immediate-release lamotrigine and more frequently than in the placebo group.
Body as a Whole: Chest pain.
Digestive: Rectal hemorrhage, peptic ulcer.
Metabolic and Nutritional: Weight decrease, peripheral edema.
Nervous: Hypesthesia, libido increase, decreased reflexes.
Respiratory: Epistaxis, dyspnea.
Skin and Appendages: Contact dermatitis, dry skin, sweating.
Special Senses: Vision abnormality.
Urogenital (female patients only): Dysmenorrhea.
Other Clinical Trial Experience
Immediate-release lamotrigine has been administered to 6,694 individuals for whom complete adverse reaction data was captured during all clinical trials, only some of which were placebo controlled.
Adverse reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions:
Cardiovascular System: Infrequent: Hypertension, palpitations, postural hypotension, syncope, tachycardia, vasodilation.
Dermatological: Infrequent: Acne, alopecia, hirsutism, maculopapular rash, urticaria.
Rare: Leukoderma, multiforme erythema, petechial rash, pustular rash.
Digestive System: Infrequent: Dysphagia, liver function tests abnormal, mouth ulceration.
Rare: Gastrointestinal hemorrhage, hemorrhagic colitis, hepatitis, melena, stomach ulcer.
Endocrine System: Rare: Goiter, hypothyroidism.
Hematologic and Lymphatic System: Infrequent: Ecchymosis, leukopenia.
Rare: Anemia, eosinophilia, fibrin decrease, fibrinogen decrease, iron deficiency anemia, leukocytosis, lymphocytosis, macrocytic anemia, petechia, thrombocytopenia.
Metabolic and Nutritional Disorders: Infrequent: Aspartate transaminase increased.
Rare: Alcohol intolerance, alkaline phosphatase increase, alanine transaminase increase, bilirubinemia, gamma glutamyl transpeptidase increase, hyperglycemia.
Musculoskeletal System: Rare: Muscle atrophy, pathological fracture, tendinous contracture.
Nervous System: Frequent: Confusion.
Infrequent: Akathisia, apathy, aphasia, depersonalization, dysarthria, dyskinesia, euphoria, hallucinations, hostility, hyperkinesia, hypertonia, libido decreased, memory decrease, mind racing, movement disorder, myoclonus, panic attack, paranoid reaction, personality disorder, psychosis, stupor.
Rare: Choreoathetosis, delirium, delusions, dysphoria, dystonia, extrapyramidal syndrome, hemiplegia, hyperalgesia, hyperesthesia, hypokinesia, hypotonia, manic depression reaction, neuralgia, paralysis, peripheral neuritis.
Respiratory System: Rare: Hiccup, hyperventilation.
Special Senses: Frequent: Amblyopia.
Infrequent: Abnormality of accommodation, conjunctivitis, dry eyes, ear pain, photophobia, taste perversion, tinnitus.
Rare: Deafness, lacrimation disorder, oscillopsia, parosmia, ptosis, strabismus, taste loss, uveitis, visual field defect.
Urogenital System: Infrequent: Abnormal ejaculation, hematuria, impotence, menorrhagia, polyuria, urinary incontinence.
Rare: Acute kidney failure, breast neoplasm, creatinine increase, female lactation, kidney failure, kidney pain, nocturia, urinary retention, urinary urgency.
3.3Postmarketing Experience with Immediate-Release Lamotrigine
The following adverse reactions have been identified during postapproval use of immediate-release lamotrigine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic
Agranulocytosis, hemolytic anemia, lymphadenopathy not associated with hypersensitivity disorder, pseudolymphoma.
Gastrointestinal
Esophagitis.
Hepatobiliary Tract and Pancreas
Pancreatitis.
Immunologic
Hypogammaglobulinemia, lupus-like reaction, vasculitis.
Lower Respiratory
Apnea.
Musculoskeletal
Rhabdomyolysis has been observed in patients experiencing hypersensitivity reactions.
Nervous System
Aggression, exacerbation of Parkinsonian symptoms in patients with pre-existing Parkinson’s disease, tics.
Non-site Specific
Progressive immunosuppression.
Renal and Urinary Disorders
Tubulointerstitial nephritis (has been reported alone and in association with uveitis).
4DRUG INTERACTIONS
Significant drug interactions with lamotrigine are summarized in this section. Additional details of these drug interaction studies, which were conducted using immediate-release lamotrigine, are provided in the Clinical Pharmacology section
Uridine 5´-diphospho-glucuronyl transferases (UGT) have been identified as the enzymes responsible for metabolism of lamotrigine. Drugs that induce or inhibit glucuronidation may, therefore, affect the apparent clearance of lamotrigine. Strong or moderate inducers of the cytochrome P450 3A4 (CYP3A4) enzyme, which are also known to induce UGT, may also enhance the metabolism of lamotrigine.
Those drugs that have been demonstrated to have a clinically significant impact on lamotrigine metabolism are outlined in Table 13. Specific dosing guidance for these drugs is provided in the Dosage and Administration section, and, for women taking estrogen-containing products, including oral contraceptives, in the Warnings and Precautions section
Effect of LAMICTAL XR on Organic Cationic Transporter 2 Substrates
Lamotrigine is an inhibitor of renal tubular secretion via organic cationic transporter 2 (OCT2) proteins
5DESCRIPTION
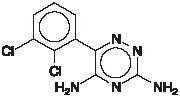
LAMICTAL XR (lamotrigine), an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Lamotrigine’s chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-
LAMICTAL XR extended-release tablets are supplied for oral administration as 25-mg (yellow with white center), 50-mg (green with white center), 100-mg (orange with white center), 200-mg (blue with white center), 250-mg (purple with white center), and 300-mg (gray with white center) tablets. Each tablet contains the labeled amount of lamotrigine and the following inactive ingredients: glycerol monostearate, hypromellose, lactose monohydrate; magnesium stearate; methacrylic acid copolymer dispersion, polyethylene glycol 400, polysorbate 80, silicon dioxide (25- and 50-mg tablets only), titanium dioxide, triethyl citrate, carmine (250-mg tablet only), iron oxide black (50-, 250-, and 300-mg tablets only), iron oxide yellow (25-, 50-, and 100-mg tablets only), iron oxide red (100-mg tablet only), FD&C Blue No. 2 Aluminum Lake (200- and 250-mg tablets only). Tablets are printed with edible black ink.
LAMICTAL XR extended-release tablets contain a modified-release eroding formulation as the core. The tablets are coated with a clear enteric coat and have an aperture drilled through the coats on both faces of the tablet (DiffCORE) to enable a controlled release of drug in the acidic environment of the stomach. The combination of this and the modified-release core are designed to control the dissolution rate of lamotrigine over a period of approximately 12 to 15 hours, leading to a gradual increase in serum lamotrigine levels.
6REFERENCES
1. French JA, Wang S, Warnock B, Temkin N. Historical control monotherapy design in the treatment of epilepsy.
7HOW SUPPLIED/STORAGE AND HANDLING
LAMICTAL XR (lamotrigine) extended-release tablets
25-mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25”, unit-of-use bottles of 30 with orange caps (NDC 0173-0754-00).
50-mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”, unit-of-use bottles of 30 with orange caps (NDC 0173-0755-00).
100-mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”, unit-of-use bottles of 30 with orange caps (NDC 0173-0756-00).
200-mg, blue with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 200”, unit-of-use bottles of 30 with orange caps (NDC 0173-0757-00).
250-mg, purple with a white center, caplet-shaped, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 250”, unit-of-use bottles of 30 with orange caps (NDC 0173-0781-00).
300-mg, gray with a white center, caplet-shaped, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 300”, unit-of-use bottles of 30 with orange caps (NDC 0173-0761-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Taking Valproate (Blue XR Kit)
25-mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25” and 50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; blister pack of 21/25-mg tablets and 7/50-mg tablets (NDC 0173-0758-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Taking Carbamazepine, Phenytoin, Phenobarbital, or Primidone, and Not Taking Valproate (Green XR Kit)
50-mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; 100 mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”; and 200 mg, blue with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 200”; blister pack of 14/50-mg tablets, 14/100-mg tablets, and 7/200-mg tablets (NDC 0173-0759-00).
LAMICTAL XR (lamotrigine) Patient Titration Kit for Patients Not Taking Carbamazepine, Phenytoin, Phenobarbital, Primidone, or Valproate (Orange XR Kit)
25-mg, yellow with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 25”; 50 mg, green with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 50”; and 100 mg, orange with a white center, round, biconvex, film-coated tablets printed on one face in black ink with “LAMICTAL” and “XR 100”; blister pack of 14/25-mg tablets, 14/50-mg tablets, and 7/100-mg tablets (NDC 0173-0760-00).
Storage
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Rash
Prior to initiation of treatment with LAMICTAL XR, inform patients that a rash or other signs or symptoms of hypersensitivity (e.g., fever, lymphadenopathy) may herald a serious medical event and instruct them to report any such occurrence to their healthcare providers immediately.
Hemophagocytic Lymphohistiocytosis
Prior to initiation of treatment with LAMICTAL XR, inform patients that excessive immune activation may occur with LAMICTAL XR and that they should report signs or symptoms such as fever, rash, or lymphadenopathy to a healthcare provider immediately.
Multiorgan Hypersensitivity Reactions, Blood Dyscrasias, and Organ Failure
Inform patients that multiorgan hypersensitivity reactions and acute multiorgan failure may occur with LAMICTAL XR. Isolated organ failure or isolated blood dyscrasias without evidence of multiorgan hypersensitivity may also occur. Instruct patients to contact their healthcare providers immediately if they experience any signs or symptoms of these conditions
Cardiac Rhythm and Conduction Abnormalities
Inform patients that, due to its mechanism of action, LAMICTAL XR could lead to irregular or slowed heart rhythm. This risk is increased in patients with underlying cardiac disease or heart conduction problems or who are taking other medications that affect heart conduction. Patients should be made aware of and report cardiac signs or symptoms to their healthcare provider right away. Patients who develop syncope should lie down with raised legs and contact their healthcare provider
Suicidal Thinking and Behavior
Inform patients, their caregivers, and families that AEDs, including LAMICTAL XR, may increase the risk of suicidal thoughts and behavior. Instruct them to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts or behavior or thoughts about self-harm. Instruct them to immediately report behaviors of concern to their healthcare providers.
Worsening of Seizures
Instruct patients to notify their healthcare providers if worsening of seizure control occurs.
Central Nervous System Adverse Effects
Inform patients that LAMICTAL XR may cause dizziness, somnolence, and other symptoms and signs of central nervous system depression. Accordingly, instruct them neither to drive a car nor to operate other complex machinery until they have gained sufficient experience on LAMICTAL XR to gauge whether or not it adversely affects their mental and/or motor performance.
Pregnancy and Nursing
Instruct patients to notify their healthcare providers if they become pregnant or intend to become pregnant during therapy and if they intend to breastfeed or are breastfeeding an infant.
Encourage patients to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of AEDs during pregnancy. To enroll, patients can call the toll-free number 1-888-233-2334
Inform patients who intend to breastfeed that LAMICTAL XR is present in breast milk and advise them to monitor their child for potential adverse effects of this drug. Discuss the benefits and risks of continuing breastfeeding.
Use of Estrogen-Containing Products, Including Oral Contraceptives
Instruct women to notify their healthcare providers if they plan to start or stop use of oral contraceptives or other female hormonal preparations (including HRT). Starting estrogen-containing products, including oral contraceptives, may significantly decrease lamotrigine plasma levels, and stopping estrogen-containing oral contraceptives (including the pill-free week) may significantly increase lamotrigine plasma levels
Discontinuing LAMICTAL XR
Instruct patients to notify their healthcare providers if they stop taking LAMICTAL XR for any reason and not to resume LAMICTAL XR without consulting their healthcare providers.
Aseptic Meningitis
Inform patients that LAMICTAL XR may cause aseptic meningitis. Instruct them to notify their healthcare providers immediately if they develop signs and symptoms of meningitis such as headache, fever, nausea, vomiting, stiff neck, rash, abnormal sensitivity to light, myalgia, chills, confusion, or drowsiness while taking LAMICTAL XR.
Potential Medication Errors
To avoid a medication error of using the wrong drug or formulation, strongly advise patients to visually inspect their tablets to verify that they are LAMICTAL XR each time they fill their prescription
LAMICTAL XR is a trademark owned by or licensed to the GSK group of companies. The other brand listed is a trademark owned by or licensed to its owner and is not owned by or licensed to the GSK group of companies. The maker of this brand is not affiliated with and does not endorse the GSK group of companies or its products.
GlaxoSmithKline
Durham, NC 27701
©2025 GSK group of companies or its licensor.
LXR:28PI