Brand Name
Cosopt
Generic Name
Dorzolamide
View Brand Information FDA approval date: October 28, 2008
Classification: Carbonic Anhydrase Inhibitor
Form: Solution
What is Cosopt (Dorzolamide)?
Dorzolamide hydrochloride ophthalmic solution, USP is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma. Dorzolamide hydrochloride ophthalmic solution, USP is a carbonic anhydrase inhibitor indicated for the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
Top Global Experts
Save this treatment for later
Not sure about your diagnosis?
Tired of the same old research?
Related Clinical Trials
Efficacy and Tolerability of Simbrinza and Rocklatan vs. Cosopt
Summary: A randomized, multi-site, parallel-group, prospective study of patients who are adults with a diagnosis of mild to moderate open-angle glaucoma (OAG), currently on an on-label use of combination topical medication of Cosopt and Latanoprost for a minimum of 1 month.
Related Latest Advances
Brand Information
COSOPT (dorzolamide hydrochloride and timolol maleate)
1INDICATIONS AND USAGE
COSOPT
2DOSAGE AND ADMINISTRATION
The dose is one drop of COSOPT in the affected eye(s) two times daily.
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart
3DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing dorzolamide 20 mg/mL (2%) equivalent to 22.26 mg/mL of dorzolamide hydrochloride, and timolol 5 mg/mL (0.5%) equivalent to 6.83 mg/mL of timolol maleate.
4OVERDOSAGE
Symptoms consistent with systemic administration of beta-blockers or carbonic anhydrase inhibitors may occur, including electrolyte imbalance, development of an acidotic state, dizziness, headache, shortness of breath, bradycardia, bronchospasm, cardiac arrest and possible central nervous system effects. Serum electrolyte levels (particularly potassium) and blood pH levels should be monitored.
A study of patients with renal failure showed that timolol did not dialyze readily.
5DESCRIPTION
COSOPT (dorzolamide hydrochloride and timolol maleate ophthalmic solution) is the combination of a topical carbonic anhydrase inhibitor and a topical beta-adrenergic receptor blocking agent.
Dorzolamide hydrochloride is described chemically as: (4
Its empirical formula is C
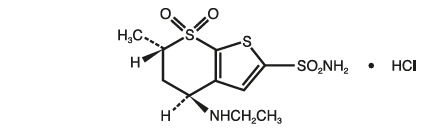
Dorzolamide hydrochloride has a molecular weight of 360.91. It is a white to off-white, crystalline powder, which is soluble in water and slightly soluble in methanol and ethanol.
Timolol maleate is described chemically as: (-)-1-(
Its molecular formula is C
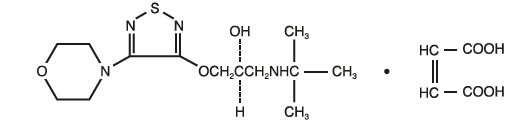
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate is stable at room temperature.
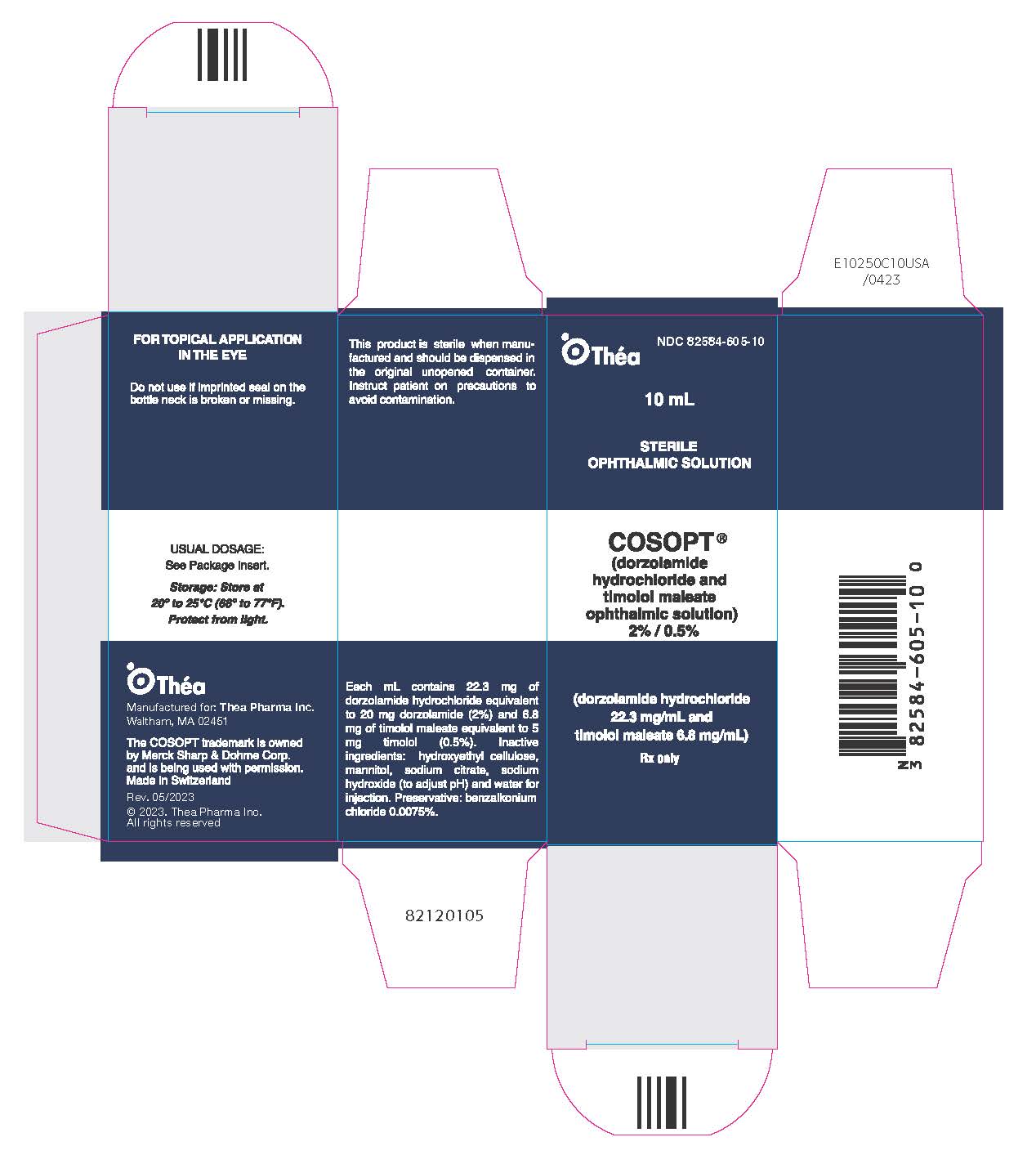
COSOPT is supplied as a sterile, clear, colorless to nearly colorless, isotonic, buffered, slightly viscous, aqueous solution. The pH of the solution is approximately 5.65, and the osmolarity is 242 to 323 mOsM. Each mL of COSOPT contains 20 mg dorzolamide (equivalent to 22.26 mg of dorzolamide hydrochloride) and 5 mg timolol (equivalent to 6.83 mg timolol maleate). Inactive ingredients are sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, and water for injection. Benzalkonium chloride 0.0075% is added as a preservative.
6CLINICAL STUDIES
Clinical studies of 3 to 15 months duration were conducted to compare the IOP-lowering effect over the course of the day COSOPT twice daily (dosed morning and bedtime) to individually and concomitantly administered 0.5% timolol twice daily and 2% dorzolamide twice and three times daily. The IOP-lowering effect of COSOPT twice daily was greater (1 to 3 mmHg) than that of monotherapy with either 2% dorzolamide three times daily or 0.5% timolol twice daily. The IOP-lowering effect of COSOPT twice daily was approximately 1 mmHg less than that of concomitant therapy with 2% dorzolamide three times daily and 0.5% timolol twice daily.
Open-label extensions of two studies were conducted for up to 12 months. During this period, the IOP-lowering effect of COSOPT twice daily was consistent during the 12 month follow-up period.
7HOW SUPPLIED/STORAGE AND HANDLING
COSOPT
NDC 82584-605-10 10 mL capacity bottle.
8PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-Approved patient labeling (Patient Information and Instructions for Use).
9PATIENT INFORMATION COSOPT ®(CO-sopt) (dorzolamide hydrochloride and timolol maleate ophthalmic solution) for topical ophthalmic use
What is COSOPT?
COSOPT is a prescription eye drop solution that contains two medicines, dorzolamide hydrochloride called an ophthalmic carbonic anhydrase and timolol maleate called a beta-blocker.
COSOPT is used to lower high pressure in the eye in people with open-angle glaucoma or ocular hypertension when a beta-blocking medicine alone does not work to control the eye pressure. It is not known if COSOPT is safe and effective in children 2 years of age and younger.
Do not use COSOPT if you:
- have or have had asthma.
- have chronic obstructive pulmonary disease (COPD) which emphysema, chronic bronchitis or both.
- have heart problems including a slow heartbeat, heart block, heart failure, or your heart muscle suddenly becomes weak due to a severe heart attack or other heart problem that caused heart damage (cardiogenic shock).
- are allergic to any of the ingredients in COSOPT. See the end of this Patient Information leaflet for a complete list of ingredients in COSOPT.
Before using COSOPT, tell your healthcare provider about all your medical conditions, including if you:
- have or have had allergies to sulfa drugs
- have a history of anaphylactic reactions to allergens
- have atopy (genetic disposition to develop allergic reactions)
- have or have had muscle weakness or myasthenia gravis
- have diabetes
- have thyroid disease
- have or have had kidney or liver problems
- plan to have any type of surgery
- wear contact lenses
- are using any other eye drops
- have an eye infection or eye trauma
- are pregnant or plan to become pregnant. It is not know if COSOPT will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while using COSOPT. You and your healthcare provider will decide if you should use COSOPT while you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if COSOPT passes into breastmilk. Talk to your healthcare provider about the best way to feed your baby while using COSOPT.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
COSOPT may affect the way medicines work, and other medicines may affect how COSOPT works. Do not start a new medicine without first talking to your healthcare provider.
Ask your healthcare provider or pharmacist for a list of medicines you are using, if you are not sure. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use COSOPT?
- See the complete
- Use COSOPT exactly as your healthcare provider tells you.
- Use 1 drop of COSOPT in the affected eye or both eyes if needed, 2 times each day. 1 drop in the morning and 1 drop in the evening.
- If you are using COSOPT with another eyedrop,
- If you have eye surgery or have any problems with your eye such as trauma or an infection, talk to your healthcare provider about continuing to use the bottle (multidose) that contains COSOPT.
- COSOPT contains a preservative called benzalkonium chloride. The preservative may be absorbed by soft contact lenses. If you wear contact lenses, remove them before using COSOPT. The lenses can be placed back into your eyes 15 minutes after using COSOPT.
- Do not touch your eye or eyelid with the dropper tip. Eye medicines, not handled the right way, can become contaminated by bacteria that can cause eye infections. Serious damage to the eye and followed by loss of vision may happen from using contaminated eye medicines. If you think your COSOPT medicine may be contaminated, or if you develop an eye infection, contact your healthcare provider right away about continuing to use your bottle of COSOPT.
- If you use too much COSOPT you may have dizziness, headaches, shortness of breath, slow heartbeats, or problems breathing. If you have any of these symptoms call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of COSOPT?
COSOPT may cause serious side effects, including:
- severe breathing problems. These breathing problems can happen in people who have asthma, chronic obstructive pulmonary disease, or heart failure and can cause death. Tell your healthcare provider right away if you have breathing problems while using COSOPT.
- heart failure. This can happen in people who already have heart failure and in people who have never had heart failure before. Tell your healthcare provider right away if you get any of these symptoms of heart failure while taking COSOPT:
- shortness of breath
- irregular heartbeat (palpitations)
- swelling of your ankles or feet
- sudden weight gain
- serious sulfa (sulfonamide) reactions. Serious reactions including death can happen in people who are allergic to sulfonamide medicines like one of the medicines in COSOPT. Other serious reactions can include:
- severe skin reactions
- liver problems
- blood problems
- swelling of your face, lips, mouth, or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest
- sweating
- increased allergic reactions. People who have a genetic history of developing allergies (atopy) or who have a history of severe anaphylactic reactions from different allergens may have increased allergic reactions while taking beta-blockers, like one of the medicines in COSOPT. Your usual dose of epinephrine used to treat your anaphylactic reactions may not work as well. Stop using COSOPT and call your healthcare provider or get emergency help right away if you get any of these symptoms of an allergic reaction:
- swelling of your face, lips, mouth or tongue
- trouble breathing
- wheezing
- severe itching
- skin rash, redness, or swelling
- dizziness or fainting
- fast heartbeat or pounding in your chest
- sweating
- worsening muscle weakness. Muscle weakness symptoms including double vision or drooping eyelids can happen while using COSOPT. Muscle weakness can get worse in people who already have problems with muscle weakness like myasthenia gravis.
- swelling of eye. Some people with low counts of certain types of cells in the eye have developed corneal edema when using COSOPT. Call your healthcare provider if you have swelling in your eyes.
The most common side effects of COSOPT include:
- eye burning
- eye stinging
- eye redness
- blurred vision
- eye tearing
- eye itching
- a bitter, sour, or unusual taste after putting in your eyedrops
These are not all the possible side effects of COSOPT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store COSOPT?
- Store at 68° to 77°F (20° to 25°C).
- Protect from light.
- Do not use COSOPT after the expiration date on the bottle.
Keep COSOPT and all medicines out of the reach of children.
General information about the safe and effective use of COSOPT.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use COSOPT for a condition for which it was not prescribed. Do not give COSOPT to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about COSOPT that is written for health professionals.
What are the ingredients in COSOPT?
Active ingredients: dorzolamide hydrochloride and timolol maleate
Inactive ingredients: sodium citrate, hydroxyethyl cellulose, sodium hydroxide, mannitol, water for injection and benzalkonium chloride added as a preservative.
This Patient Package Information has been approved by the U.S. Food and Drug Administration | 11/2020
10INSTRUCTIONS FOR USE COSOPT ®(CO-sopt) (dorzolamide hydrochloride and timolol maleate ophthalmic solution) for topical ophthalmic use
Read this Instructions for Use before you start using COSOPT and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information:
- COSOPT is for use in the eye.
- If you are using COSOPT with another eyedrop,
- If you wear contact lenses, remove them before using COSOPT. The lenses can be placed back into your eyes
- Do not touch your eye or eyelid with the dropper tip. Eye medicines, not handled the right way, can become contaminated by bacteria that can cause eye infections. Serious damage to the eye and followed by loss of vision may happen from using contaminated eye medicines. If you think your COSOPT medicine may be contaminated, or if you develop an eye infection, contact your healthcare provider right away about continuing to use your bottle of COSOPT.
- Wash your hands before each use to make sure you do not infect your eyes while using COSOPT.
- Before using the eyedrops for the first time, be sure the Safety Seal around the cap is not broken. If the Safety Seal is broken, call your pharmacist to get a new bottle of COSOPT.
- The dropper tip is made to give a single drop of COSOPT.
- After you have used all of your doses of COSOPT, there will be some COSOPT left in the bottle.
- There is an extra amount of COSOPT that has been added to the bottle. You will get the full amount of COSOPT that your doctor prescribed.
- Do not try to remove the extra COSOPT medicine from the bottle.
This Instructions for Use has been approved by the U.S. Food and Drug Administration | 11/2020
Manufactured for:
© 2023. Thea Pharma Inc. All rights reserved
Rev. 05/2023
Revised: 5/2023
Thea Pharma Inc.
11PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
NDC 82584-605-10
Théa
10 mL
STERILE
COSOPT
(dorzolamide hydrochloride
Rx only