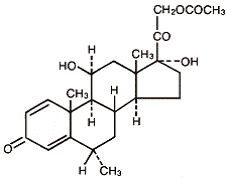
MethylPREDNISolone Acetate
What is Depo-Medrol (MethylPREDNISolone Acetate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: This phase III trial studies whether inotuzumab ozogamicin added to post-induction chemotherapy for patients with High-Risk B-cell Acute Lymphoblastic Leukemia (B-ALL) improves outcomes. This trial also studies the outcomes of patients with mixed phenotype acute leukemia (MPAL), and B-lymphoblastic lymphoma (B-LLy) when treated with ALL therapy without inotuzumab ozogamicin. Inotuzumab ozogamicin ...
Summary: TReatment for ImmUne Mediated PathopHysiology (TRIUMPH) is a multi-center, three arm, randomized, controlled trial of immunosuppressive therapy for children with acute liver failure. The study will determine if suppressing inflammatory responses with either corticosteroids or equine anti-thymocyte globulin therapy improves survival for children with this rare, life-threatening condition.
Summary: The objective of this phase 2 study is to investigate the efficacy of Dexamethasone sodium phosphate (DEX) plus Methylprednisolone acetate (MPA) in combination with plain bupivacaine (B) compared with Liposomal Bupivacaine (LB) in combination with plain bupivacaine on post-surgical pain control among patients undergoing unilateral total knee arthroplasty (TKA) to asses if perineural B-DEX-MPA will...
Related Latest Advances
Brand Information
NDC 0009-0274-01
(methylPREDNISolone acetate
injectable suspension, USP)
Rx only

Multidose Vial
(methylPREDNISolone
acetate injectable
suspension, USP)
as a Preservative

NDC 0009-0280-02
(methylPREDNISolone acetate
injectable suspension, USP)
(40 mg/mL)

(methylPREDNISolone acetate injectable suspension, USP)
(40 mg/mL)

NDC 0009-0306-02
(methylPREDNISolone acetate
injectable suspension, USP)
(80 mg/mL)

Multidose Vial
(methylPREDNISolone
acetate injectable
suspension, USP)
(80 mg/mL)
as a Preservative