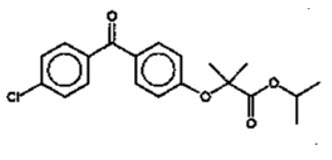
Fenofibrate
What is Lipofen (Fenofibrate)?
Approved To Treat
Top Global Experts
Related Clinical Trials
Summary: The aim of this multi-site randomized control trial will be is to assess the impact Systematic lighting on circadian rhythm entrainment, Inflammation, Neutropenic Fever and Symptom Burden among Multiple Myeloma Patients undergoing Autologous Stem Cell Transplantation. To achieve this aim, 200 multiple myeloma patients will receive one of two different light-treatments that are designed to promote ...
Summary: In this study, the investigators want to assess the effects of a new beverage containing whey protein and glycerol (two different dosages) on hydration status in healthy adults when compared to the control (water). This is a single-center, double-blinded, 3 arm cross-over randomized controlled study looking to enroll 45 healthy adult participants. The study will be performed at the Clinical Innova...
Summary: This study aims to determine the efficacy of 36 months once-daily fenofibrate in preventing clinically-detectable recurrence of primary sclerosing cholangitis after liver transplantation, compared with a historical control cohort that was not treated with
Related Latest Advances
Brand Information
- to reduce triglyceride (TG) levels in adults with severe hypertriglyceridemia (TG greater than or equal to 500 mg/dL).
- to reduce elevated low-density lipoprotein cholesterol (LDL-C) in adults with primary hyperlipidemia when use of recommended LDL-C lowering therapy is not possible.
- 50 mg: Size 3 white opaque gelatin capsule imprinted "G 246" and "50" in black ink.
- 150 mg: Size 1 white opaque gelatin capsule imprinted "G 248" and "150" in green ink.
- Severe renal impairment, including those with end-stage renal disease (ESRD) and those receiving dialysis
- Active liver disease, including those with unexplained persistent liver function abnormalities
- Pre-existing gallbladder disease
- Hypersensitivity to fenofibrate, fenofibric acid, or any of the excipients in LIPOFEN. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with fenofibrate
- Mortality and coronary heart disease morbidity
- Hepatoxicity
- Myopathy and Rhabdomyolysis
- Increases in Serum Creatinine
- Cholelithiasis
- Increased Bleeding Risk with Coumarin Anticoagulants
- Pancreatitis
- Hematologic Changes
- Hypersensitivity reactions
- Venothromboembolic disease
- Paradoxical Decreases in HDL Cholesterol Levels